Architects of Protection: How the Placenta and Barrier Tissues Safeguard Development
We study how the cells that build our organs also protect them, beginning in the womb. Our research focuses on the placenta, a powerful and often overlooked organ that shields the fetus from harm while shaping immune and developmental health. We investigate how placental barrier cells respond to infections and complications, and how similar defenses function in the gut, brain, and reproductive tract. By exploring how early-life tissues fight infection, from pregnancy through infancy, we aim to uncover new ways to protect mothers and babies from disease.
Trophoblast organoid derived from rhesus macaque placental tissue.
Exploring the Maternal-Fetal Interface Across Evolution—One Organoid at a Time
What can we learn about pregnancy by studying a bat, a lemur, or a human side by side? A lot more than you might think. Our lab uses organoids, miniature, lab-grown versions of the placenta, to explore how different mammals have evolved distinct strategies to support healthy pregnancies. These models allow us to recreate and study the maternal-fetal interface, where the mother’s body and the developing fetus communicate, share nutrients, and defend against infection. By comparing placental organoids from humans, bats, non-human primates, and other mammals, we can identify species-specific adaptations that have emerged over millions of years of evolution. These insights help us understand not only how pregnancy works in extreme environments like high-altitude habitats or during flight in bats, but also why some species (including humans) may be more vulnerable to pregnancy complications or infections. This evolutionary approach, powered by cutting-edge stem cell science, opens up exciting possibilities: better models of human pregnancy, deeper understanding of immune protection during gestation, and even new strategies for improving reproductive success in endangered species. With every organoid we grow, we take a step closer to decoding the incredible biology that makes mammalian life possible.
Human stem cell-derived cerebral organoid.
Enterovirus Infections: Unraveling a Hidden Threat
Enteroviruses are a widespread but often overlooked group of viruses that cause serious illnesses including viral meningitis, hand-foot-and-mouth disease, respiratory infections, and even heart inflammation. Yet despite their impact on human health, we still know surprisingly little about how these viruses cause disease at the cellular level. Our lab is focused on solving this mystery. We are working to identify the cellular entry points—the receptors that enteroviruses use to infect human tissues—and to understand how these viruses behave differently in the brain, gut, lungs, and heart. This work is especially critical for emerging and clinically important viruses such as coxsackievirus B (CVB), echoviruses, enterovirus 71 (EV71), enterovirus D68 (EV-D68), and EV-D70. To uncover how these viruses interact with the human body, we use state-of-the-art tools: stem cell–derived organoids, miniaturized models of human organs grown in the lab, and animal models that replicate how enteroviruses spread and cause disease. These platforms allow us to track infections in real time, understand how different tissues respond, and test potential therapies in highly realistic systems. By combining basic virology with cutting-edge modeling, our goal is to develop better strategies to predict, prevent, and treat enterovirus infections—especially in infants, children, and vulnerable populations who are most at risk.
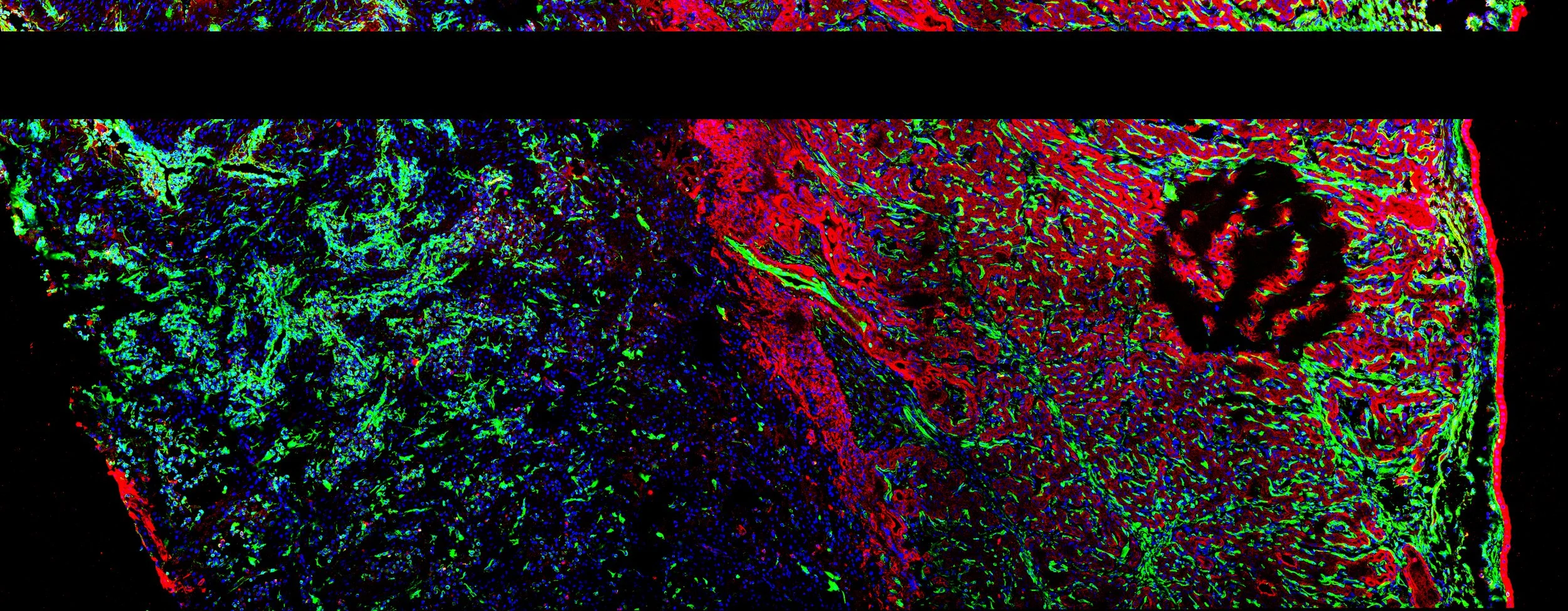
Human trophoblast organoid infected with human cytomegalovirus (HCMV).